Using the treatments
See individual product information and specialised references.
Advise patients to reduce their caffeine intake by half when stopping tobacco smoking.
NRT
Australian guidelines recommend the following initial NRT doses for smoking cessation9:
- Smokes within 30 minutes of waking and smokes >10 cigarettes a day
- highest-strength patch + highest-strength gum OR highest-strength lozenge OR 1 mg oral spray
- Smokes within 30 minutes of waking and smokes ≤10 cigarettes a day OR smokes more than 30 minutes after waking and smokes >10 cigarettes a day
- highest-strength patch + lowest-strength gum OR lowest-strength lozenge OR 1 mg oral spray
- Smokes more than 30 minutes after waking and smokes ≤10 cigarettes a day
- lowest-strength gum OR lowest-strength lozenge OR 1 mg oral spray.
Titrate the dose according to the patient’s withdrawal symptoms. Underdosing can undermine a patient’s confidence in treatment. Patients with high nicotine dependence may benefit from the use of two patches at the same time (see ‘Clinical features’). However, the evidence supporting the use of two patches is inconclusive.9,23
Premature discontinuation of NRT can lead to relapse. Tapering the dose of NRT at the completion of a course of treatment does not influence successful long-term smoking cessation. Patients who have successfully stopped smoking after an initial 8-week course of NRT may consider a follow-up course. The optimal duration of NRT has not been established.9,23
Food and drink can reduce buccal absorption of nicotine. Avoid acidic beverages (e.g. coffee, soft drinks) for 15 minutes before use of sublingual/buccal NRT formulations, and avoid eating or drinking while using sublingual/buccal NRT formulations.17
Using the highest-strength patch for 2 weeks before stopping smoking completely can increase the likelihood of success. However, further research is needed.9,23
Faster-acting NRT can be used to reduce the number of cigarettes smoked each day. However, the evidence of the effectiveness of this approach is uncertain.9,17
Vapes
Advise the patient of the evidence for vapes and how to use them. This may include1,12:
- evidence
- vapes are unapproved products that have not been assessed by the TGA for their quality, safety and efficacy or performance
- nicotine vapes are not first-line pharmacotherapy for smoking or vaping cessation; there are other smoking cessation medicines that have been approved by the TGA for quality, safety and efficacy
- how to use the vaping device
- some suppliers of vaping devices have instructions about how to use the device
- avoiding smoking in combination with vaping
- continued tobacco smoking while trying to stop smoking using vapes may lead to increased nicotine consumption and/or cumulative cardiovascular adverse effects1,30,31
- how much and how often to use vapes
- the ‘dose’ of nicotine delivered from a vape depends on a number of factors including the nicotine concentration, the device used, and the inhalation technique
- there is no distinct end-point as there is with smoking (i.e. finishing and stubbing out a cigarette); the volume of vaping substance in a single pod or cartridge is far more than would typically be used in a single vape session
- duration of treatment
- the long-term effects of vaping are unknown
- vaping should only be used short-term for smoking cessation – the optimal duration of nicotine vapes has not been established
- do not continue supplying vapes without a prescription for longer than 12 weeks
- current Australian guidelines recommend a maximum treatment duration of 12 months, however, there may be instances where the prescriber and patient agree that longer-term use of a nicotine vape is needed to avoid relapse to tobacco use
- weaning strategies
- the optimal strategy to titrate down nicotine vaping use to achieve cessation has not been established. Suggestions include:
- attempting weaning or cessation after 12 weeks
- transitioning from nicotine vapes to NRT
- limiting vaping to particular times or places
- increasing the time between vapes
- tapering the dose (e.g. reducing the nicotine concentration every 2–4 weeks as well as the number of vape session per day)
- how to store and dispose of the medicine and device
- return any containers with vaping liquid (e.g. cartridges, pods) or empty containers that may contain residual nicotine to the pharmacy for disposal through the return of unwanted medicines (RUM) program
- the device should be switched off and batteries removed prior to disposal in household waste; contact local council for advice on disposal of vaping device batteries
- how to reduce the risk or impact of accidental nicotine poisoning
- using nicotine vapes with a nicotine concentration of ≤20 mg/mL
- using a closed system device
- keeping vapes out of reach of children and avoiding use of vapes in front of children
- call 000 if inadvertent exposure or ingestion of a nicotine vaping substance has occurred in a child (or adult who is experiencing signs and symptoms suggestive of nicotine overdose)32
- contact the Poisons Information Centre (13 11 26) and seek urgent medical assistance if inadvertent exposure or ingestion of a nicotine vaping substance has occurred in an adult.
Nicotine vape doses
There is limited evidence for starting nicotine concentrations for the treatment of nicotine dependence. Nicotine vapes contain nicotine in either free-base (bioactive form) or salt form. Free-base and salt products with the same nicotine concentration are not directly interchangable.1 See Table 7 for suggested reasonable starting concentrations for new and current users of nicotine vapes. Review the appropriateness of the nicotine vape concentration at all follow-ups and adjust according to the patient’s needs.
Table 7 Nicotine vape suggested starting concentration
|
|
|
|
Current nicotine vape users*
|
|
|
Lower nicotine dependence(Smokes more than 30 minutes after waking and smokes ≤10 cigarettes a day)
|
Higher nicotine dependence(Smokes within 30 minutes of waking or smokes >10 cigarettes a day)
|
|
|
|
|
|
Base on current usage
• if currently using an open system, switch to a closed system
• nicotine free-base and nicotine salt products with the same concentration are not directly interchangable, carefully consider appropriate dose for the patient if switching between free-base and salt
|
|
|
|
|
|
Reference: RACGP 1
* Only vapes with a nicotine concentration ≤20 mg/ml can be supplied as a Schedule 3 (Pharmacist Only) medicine. See ‘Poisons Standard schedules’ and ‘Therapeutic vaping goods’ for full list of requirements for supply of therapeutic vaping goods without a prescription.
There is limited evidence for the volume of nicotine vape liquid that should be used for the treatment of nicotine dependence. The following factors should be considered when determining an appropriate quantity for the patient:
- The dose of nicotine received by a patient using a nicotine vape varies depending on the device, type of nicotine (free-base or salt), concentration and the length and intensity of inhalation.1,33
- Patients will self-titrate to achieve the nicotine level required to satisfy their craving.1
- The safety of vapourised chemicals (including excipients, chemicals originating from devices and chemicals formed by chemical reactions with the heating element) in vapes is currently unknown.33 There are concerns that inhaling high volumes of vape liquid in low concentration products could increase risk of adverse effects due to an increased quantity of inhaled chemicals.1,26
- If the concentration of nicotine vape is too low for the patient, they may use a higher volume of vaping substance, which will increase the cost to the patient.
- A suggested reasonable quantity of nicotine vape liquid to use is 1 pod or cartridge (∼2 mL) per day. If a patients usage exceeds this, they should be referred to a medical or nurse practitioner for a higher concentration product.34
Varenicline
- Start at least 1 week before planned smoking cessation. Alternatively, the patient can start using varenicline and then stop smoking anytime between day 8 and day 35 of treatment.17
- Continue treatment for 11–23 weeks.10,17
- Swallow tablets whole.17
- There is a risk of nicotine withdrawal symptoms and an urge to smoke after varenicline is ceased; reducing the dose gradually or using a faster-acting NRT product may minimise this.10
Bupropion
- Start at least 1 week before planned smoking cessation.10,17
- Continue treatment for 7–9 weeks.10,17
- Can be used in combination with NRT (e.g. a faster-acting product to manage nicotine cravings – blood pressure should be monitored if this combination is used).10,17
- Swallow tablets whole.10
- Alcohol consumption should be minimised (or avoided) as it alters the seizure threshold and increases the risk of other adverse effects.10,17
Adverse effects
Advise the patient of the following potential adverse effects of treatment.
NRT
- Adverse effects of NRT are usually minor and transient, and some may be related to smoking cessation (e.g. sleep disturbance, dizziness, weight gain, headache).17 See Table 8 and ‘Non-pharmacological management’.
- Signs of nicotine overdose include nausea, vomiting, bradycardia and convulsions.17,35
- NRT is intended for transdermal or sublingual/buccal absorption. Swallowed nicotine may exacerbate symptoms of oesophagitis, gastritis and gastric ulcers.17
Table 8 Adverse effects of NRT
|
|
|
|
|
|
Application site skin reactions
|
Rotate application site daily
Apply 1% hydrocortisone cream to the affected area (short-term use only)
|
|
|
Vivid dreams affecting daytime functioning
|
|
|
|
Patch adhesive not sticking
|
Use adhesive skin tape to provide extra adhesion
|
|
|
Throat or mouth irritation, hiccups, cough (may be a result of swallowed nicotine)
|
Check and correct any problems with using the formulation. If adverse effects persist, consider alternative formulation
|
References: RACGP9; eMIMS17
Nicotine vapes
- cough, dry or irritated mouth and throat (throat irritation particularly with free-base form at concentrations >20 mg/mL)1
- headache1,12
- nausea1
- lung injury1
- burns1
- intentional or accidental poisoning1
- nicotine overdose – signs and symptoms include nausea, vomiting, bradycardia, convulsions
- see ‘Using the treatment’ for guidance on actions to take if intentional or accidental poisoning is suspected
- long-term adverse effects are unknown9
- adverse effects from carrier fluids (propylene glycol and glycerol), flavourings and contaminants
- flavourings have not been assessed as being safe when inhaled. Different flavourings may have different safety profiles and ingredients (e.g. the ‘mint’ flavour in two different products may be made of different ingredients)36
- known carcinogens have been found in vape aerosols.36 The extent to which vape use increases the risk of cancer is currently unknown.12 The TGO 110 recommends a maximum of 10 ppm for specified contaminants.36
Varenicline10
- gastrointestinal effects – severe nausea may be relieved by taking with food or with a reduction in dose
- increased appetite/weight
- headache
- insomnia
- abnormal dreams
- taste disturbance
Bupropion10
- gastrointestinal effects
- insomnia – taking once daily doses in the morning may reduce the chance of insomnia – when dosing increases to twice daily, separate doses by 8 hours and avoid bedtime doses
- dry mouth
- dizziness, concentration difficulties – be careful driving or operating machinery if affected
- agitation
This guideline does not list all possible adverse effects. Consult specialised references for further information.
Non-pharmacological management
Advise patients about behavioural strategies to assist them to stop smoking or vaping. Successful long-term smoking cessation is more likely if pharmacotherapy is combined with evidence-based, multi-session behavioural intervention (e.g. Quitline).9 Behavioural intervention (e.g. Quitline) is also recommended for vaping cessation.1 Patients can also use apps, such as My QuitBuddy, for ongoing support. Best practice smoking cessation support (including both pharmacotherapy and multi-session behavioural intervention) is especially important for achieving long-term smoking cessation in patients with a mental illness.9,37
Addressing barriers to smoking and vaping cessation
Discuss the patient’s individual barriers and previous attempts to stop smoking or vaping. Discuss what strategies have been helpful in past attempts.
- Explain the benefits, costs, options and accessibility of assistance that is available (e.g. pharmacotherapy, counselling services).9,38
- Discuss the health and financial benefits of smoking and vaping cessation specifically for the patient. The National Cessation Platform (quit.org.au) has useful information about the benefits that can be tailored for the patient.9
- Discuss the health implications of continuing to smoke or vape (e.g. possible lung damage).9,37
- Provide information about relaxation techniques (e.g. mindfulness, meditation, breathing exercises).9,37
- Discuss strategies to minimise weight gain (e.g. healthy eating, exercise) and advise that the health benefits of stopping smoking outweigh the risks of weight gain.9
- Suggest ways to manage high-risk social situations when first stopping smoking or vaping and practising ways of saying no when a cigarette or vape is offered.9,37
Behavioural strategies
Discuss some practical tips for stopping smoking or vaping:
- Write a list of reasons that you want to stop smoking or vaping and things you will look forward to when you stop.37
- Make a plan and set a date to stop smoking or vaping. Decide what support options will work best for you.37
- Tell family and friends of the plan to stop smoking or vaping, and explain how they can help (e.g. stopping together, not offering a cigarette or vape).37
- Practise stopping (e.g. experiment with not smoking or vaping in situations you normally would, make home and car smoke and vape-free, stop carrying a lighter).37
- Identify situations and triggers that make you want to smoke or vape (e.g. coffee, alcohol, friends who smoke or vape), and plan for these.9,37
- Prepare for healthy eating (e.g. buy healthy snacks before stop day).37
- Reduce coffee and tea intake when you first stop smoking, to prevent caffeine-induced anxiety and restlessness.15 See ‘Medical, medicines and lifestyle history’. (This only applies to smoking cessation)
- The ‘4Ds’ can help to deal with cravings37
- Delay for at least 5 minutes, the urge will pass.
- Deep breathe, slowly.
- Drink water, to take time out; sip slowly.
- Do something else, to keep your hands busy.
- Exercise every day. This will help to reduce stress and weight gain.37
- Reward yourself with the money that has been saved.9,37
- If you have a cigarette or vape, it does not mean the attempt has failed. Keep trying to stop. Remind yourself of all the times you have resisted the urge to smoke or vape, follow the ‘4Ds’ and adjust your stop smoking or vaping plan.37
Support services
Refer patients to smoking and vaping cessation support services for additional support.
Quitline provide free, multi-session behavioural intervention. Quitline counsellors assess the patient’s smoking or vaping history and use motivational interviewing to help them develop a plan to stop smoking or vaping. Quitline is tailored to meet the needs of priority populations including patients living with mental illness, pregnant patients and young people. Quitline also provides Aboriginal and Torres Strait Islander counsellors, and is able to assist people with hearing or speech impairment, or people needing an interpreter.37
Pharmacists can refer patients to Quitline at www.quitcentre.org.au/referral-form. Alternatively, patients can contact Quitline by phone on 13 78 48.37
Written information
- Provide the patient with Smoking, Vaping, Nicotine Replacement Therapy and Staying a Non-smoker Self Care Fact Cards or other consumer information.
- Resources can be ordered from org.au/resource-order-form
Labelling of nicotine vaping products
- It is recommended that pharmacists dispense supplies of therapeutic vaping substances prescribed by a pharmacist under Schedule 3 supply using the same processes as dispensing Schedule 4 medicines. See ‘Professional practice guidelines for pharmacists: Nicotine dependence support; Dispensing‘.
- Attach cautionary advisory label 21 to therapeutic vaping goods that contain nicotine. See ‘Cautionary advisory labels‘.
- Inadvertent exposure to nicotine vape liquid (ingestion or absorption through skin or mucus membranes) by a person other than the patient is a cause of poisoning. See ‘Using the treatments’.
- Include the words ‘do not swallow’ and dosing information on the dispensing label.
Review
Invite the patient to contact the pharmacy if they have any questions or concerns about the medicine or advice provided.
Advise the patient to return to the pharmacy or consult a medical practitioner if they experience:
- concerning or unexpected adverse effects from treatment
- inadequate response to treatment
- difficulty using the treatment.
Suggest that patients return for follow-up within 1 week of the stop use date and for additional planned follow-up visits to review progress.9 Review can include:
- enquiring about the patient’s perspective on their progress
- identifying and addressing any concerns the patient has regarding the management plan
- working with the patient to determine whether pharmacotherapy should be continued, ceased or modified (e.g. dose titration of nicotine vapes)
- discussing behavioural strategies to help with smoking or vaping cessation and encouraging patients to use support services such as Quitline
- encouraging patients to avoid dual smoking and nicotine vaping
- reaffirming that the long-term risks of nicotine vapes are unknown (if patient is using nicotine vapes)
- discuss switching to approved smoking cessation pharmacotherapy to maintain their nicotine cessation progress and prevent relapse.
For varenicline or bupropion supplied via pharmacist prescribing:
- advise patient to return for review 2–3 weeks after commencing the pharmacotherapy (or shortly after quit date if more than 3 weeks after starting the pharmacotherapy) and at the end of the treatment course
- encourage ongoing support (e.g. from Quitline)
- measure the patient’s blood pressure at each review.
If relapse occurs, offer support and encourage further attempts. Acknowledge that it may take numerous attempts to stop smoking or vaping.

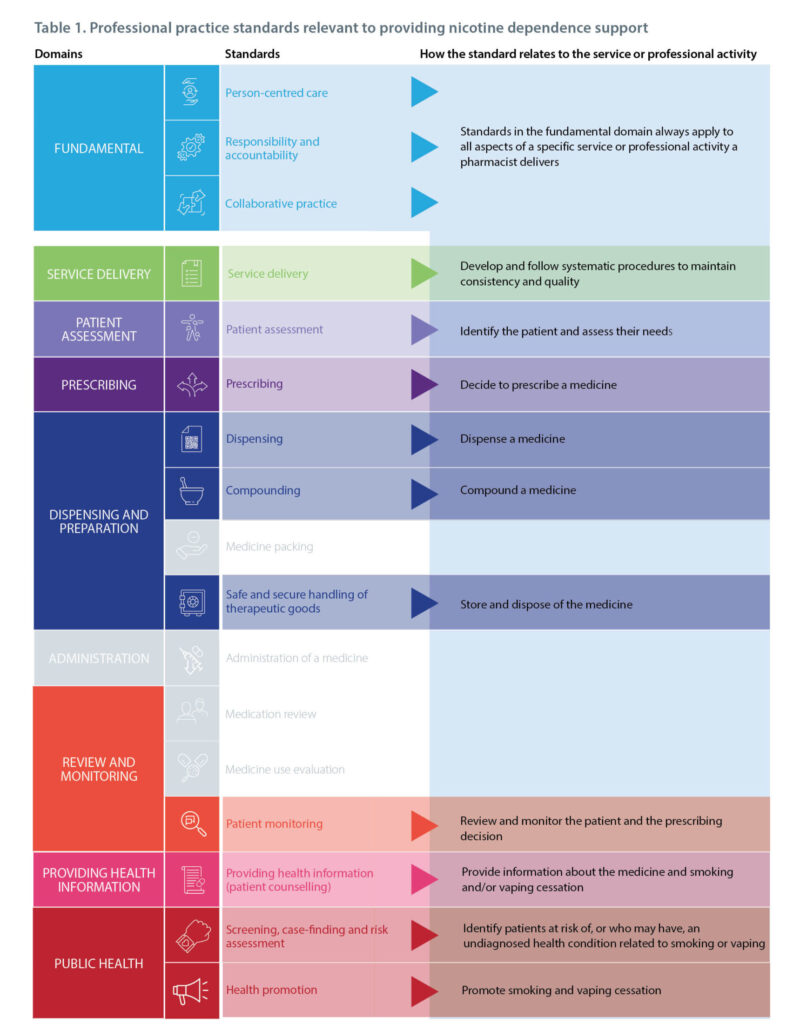



 The pharmacist provides safe and secure handling of therapeutic goods to enable access and safeguard the patient.
The pharmacist provides safe and secure handling of therapeutic goods to enable access and safeguard the patient. The pharmacist prepares compounded medicines that are safe and appropriate for the patient.
The pharmacist prepares compounded medicines that are safe and appropriate for the patient.
 The pharmacist facilitates the safe provision of a prescribed therapeutic good, according to a valid prescription or order, to treat a patient.
The pharmacist facilitates the safe provision of a prescribed therapeutic good, according to a valid prescription or order, to treat a patient. The pharmacist critically appraises information to provide accurate, evidence-based, trusted and reliable medicines and health information to meet the needs of the patient, group of people and members of the healthcare team.
The pharmacist critically appraises information to provide accurate, evidence-based, trusted and reliable medicines and health information to meet the needs of the patient, group of people and members of the healthcare team. The pharmacist uses evidence-based screening, case-finding and risk assessment methods to identify people at increased risk of, or who may have, an undiagnosed health condition.
The pharmacist uses evidence-based screening, case-finding and risk assessment methods to identify people at increased risk of, or who may have, an undiagnosed health condition. The pharmacist collaboratively monitors patient outcomes and supports patients to self-monitor their condition and prevent complications.
The pharmacist collaboratively monitors patient outcomes and supports patients to self-monitor their condition and prevent complications.

