The Digital Health Guideline Supplement: Real Time Prescription Monitoring (RTPM) describes the professional, regulatory and ethical obligations of community pharmacists when performing their professional clinical roles and interacting with the RTPM system.
These guidelines provide advice to pharmacists on expected professional practice when using the RTPM system, in order to provide safe supplies of monitored medicines to consumers, while continuing to meet legal and ethical obligations relating to data access and stewardship.
The guidelines should be used as a tool to support practice and professional decision making, and ensure that consumers’ needs, beliefs and preferences are met. The guidelines can be used as an educational resource to inform quality assurance processes and provide support in meeting legal requirements and ethical considerations.
Scope
The Digital Health Guideline Supplement: Real Time Prescription Monitoring (RTPM) is applicable to practising pharmacists who interact with the RTPM system.
Guidelines produced by the Pharmaceutical Society of Australia (PSA) are not definitive statements of correct procedure but represent agreements by experts in the field. The guidelines do not set a prescribed course of action or a mandatory standard to which pharmacists must adhere.
This guideline is not a technical guide on how to operate the RTPM system; rather it provides guidance on the professional and ethical behaviours for pharmacists when interacting with the RTPM in the delivery of health services.
Pharmacists are expected to exercise professional judgement when adapting the guidance provided in this guideline to specific circumstances.
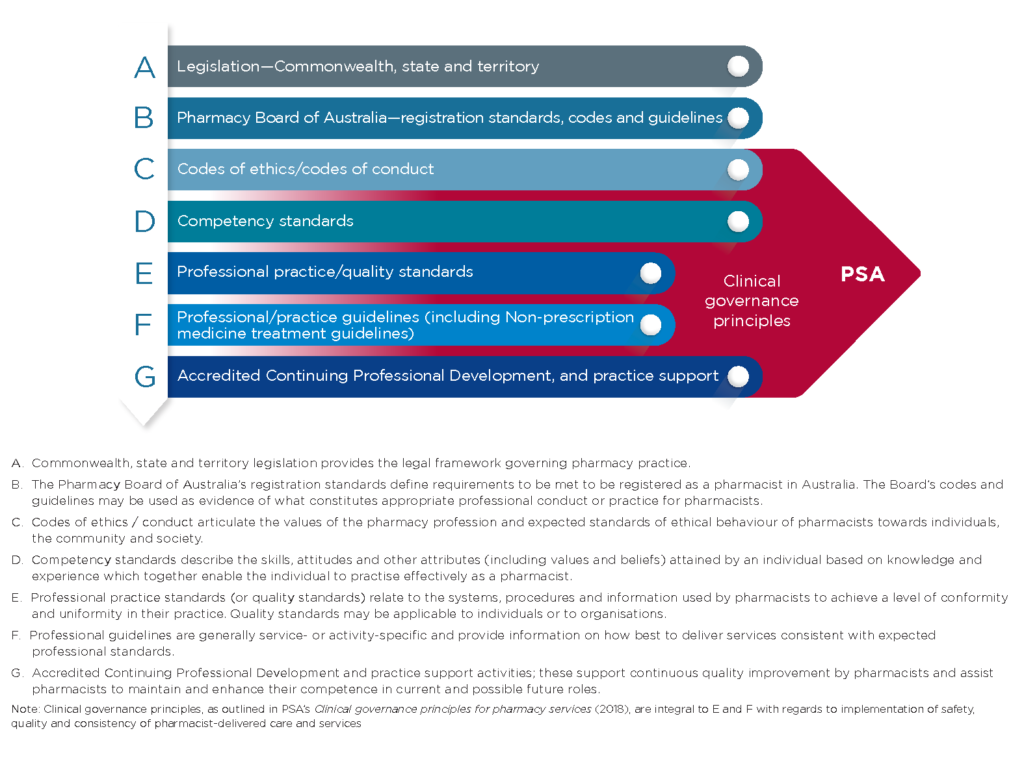
This guideline sits within a broader hierarchy of guidance underpinning and supporting the practice of pharmacists working with digital health systems (Figure1).
Acknowledgements
It is important to review this guideline in conjunction with the current versions of the:
- Pharmacy Board of Australia Code of Conduct for Pharmacists
- National Competency Standards Framework for Pharmacists in Australia
- PSA Code of Ethics for Pharmacists
- PSA Dispensing Practice Guidelines
- PSA Professional Practice Standards
- PSA Digital Health Guidelines
- Australian Commission on Safety and Quality in Health Care National Guidelines for On-Screen Display of Medicines Information (2017)